The immune system is highly specialized, and defends the body very effectively from foreign invaders. But what happens if the enemy acts like a double agent? Then the immune cells cannot see their targets, because they appear to be normal body cells. Although cancer cells are often surrounded by immune cells, they continue to grow and spread throughout the body. This paradox was first described 50 years ago by two Swedish immunologists, Karl Erik and Ingegerd Hellstroem. The reason that cancer cells survive is because they can build such powerful barriers that the immune system fails to fight them:
- Sometimes they do not display enough differences to be recognized, as they develop from “normal” cells.
- Sometimes they get caught by the immune cells, but the response is not strong enough to destroy them.
- Sometimes they release substances that hinder the immune response.
Stimulating the immune system to battle tumors is the scope of cancer immunotherapy. “Cancer immunotherapy serves a dual purpose,” explains Prof. Dr. med. Thomas Wölfel from Internal Medicine III of the University Medical Center Mainz. “On the one hand, we try to release the existing immune responses, using for example, checkpoint inhibitors or antigen-based cancer vaccines that do not target the tumor itself, but take away immunological barriers. On the other hand, we want to give patients the effectors they lack as antibodies, or T cells that specifically target cancer cells.”
Cancer immunotherapy: a fragile balance between fighting cancer and preventing autoimmunity

Advances in cancer immunotherapy in the past decade have relied primarily on greater understanding of how the immune system recognizes and kills cancer cells. The cancer-immunity cycle delineates the different steps in this complex process (Chen and Mellman, 2013). Cancer cells are characterized by the accumulation of genetic alterations, and the loss of regulatory mechanisms. Some of these somatic mutations result in the expression of so-called “neoantigens.” Dendritic cells (DCs), the most powerful antigen-presenting cells in the body, take up antigens and migrate to lymphoid organs, where they present the antigens to T cells activating them.
These activated effector T cells then go back to the tumor site, move through the endothelial barrier, bind to the cancer cells, and kill them. In cancer patients, one or more steps of this process are often impaired. The goal of immunotherapy is to initiate and maintain an efficient cycle without causing autoimmune inflammatory responses. “The cancer-immunity cycle provides the reference points for the different immunotherapeutic approaches,” remarks Wölfel in a recent article published in Der Onkologe (Wölfel, 2017).
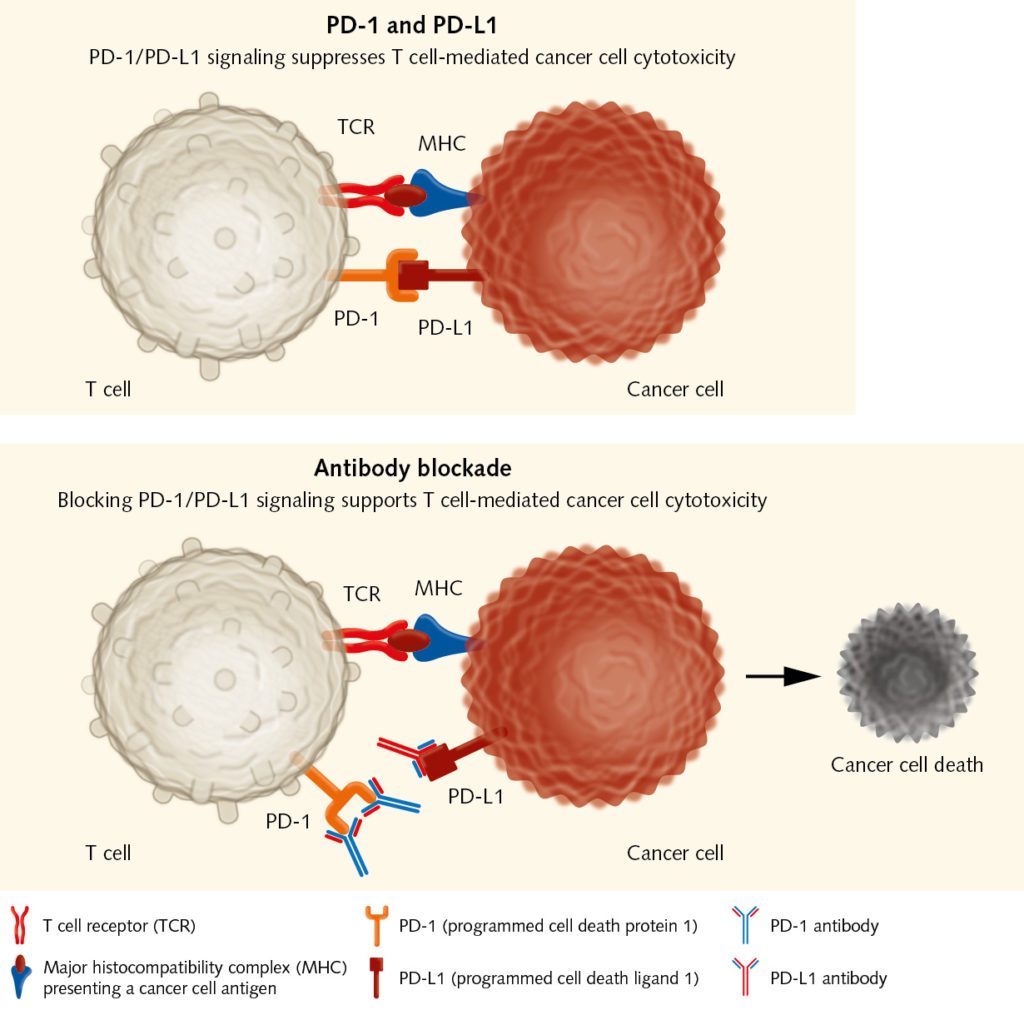
Checkpoint inhibitors: releasing the brakes on T cells
The balance between stimulatory and inhibitory signals is crucial for avoiding uncontrolled immune reactions that would damage the body. Therefore, immune cells have immune checkpoints on their surface. Those receptors – when binding to the ubiquitous ligands on body cells – prevent them from attacking “normal” cells. Tumors can also activate suppressive immune checkpoint pathways that minimize the immune response. In the late 1990, James Allison, a cancer immunologist at the University of Berkeley in California, found that the administration of antibodies at one of these checkpoints, the cytotoxic T-lymphocyte associated protein 4 (CTLA-4), eliminated tumors in mice (Leach DR et al., 1996).
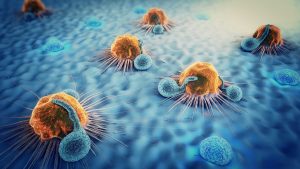
This was a huge breakthrough in cancer immunotherapy. The use of monoclonal antibodies to block inhibitory receptors on immune effector cells has been tested in many preclinical and clinical studies. The anti-CTLA-4 antibody Ipilimumab was the first-in-class drug to be approved by the FDA after clinical studies had shown that it improved survival rates in patients with advanced melanoma (Hodi et al., 2010). Besides CTLA-4, the activated programmed-cell death protein (PD-1) is another well-established target of checkpoint inhibition. Several anti-PD-1 antibodies have been developed that show positive results in many cancers, such as melanoma, lung cancer, renal cell carcinoma, head and neck cancer, bladder cancer, and Hodgkin disease (Sharon et al., 2014, Alsaab et al., 2017). These tumors express the PD-1 ligand PD-L1, which may serve as an important mechanism for suppressing tumor-reactive T cells in the tumor microenvironment. “Checkpoint inhibitors have shown that immunotherapeutic approaches can be successful in various types of solid tumor,” explains Wölfel. “They take away immune barriers and release the patient’s T cells. Researchers are now intensively looking for predictive parameters that can help in selecting the appropriate treatment for each patient.”
Cancer vaccines: stimulating the immune system to destroy cancer cells
Another approach that targets the early steps in the cancer-immunity cycle is the vaccination with tumor-associated or tumor-specific antigens. The purpose of cancer vaccines is to initiate and boost an antigen-specific T cell response when the tumor has failed to do this. Advances in technology have led to the identification of many antigens that can be targeted in cancer immunotherapy. These antigens are introduced into the patients, where they stimulate effector T cells to selectively attack cancer cells that express them. There are several ways to vaccinate the patients. Dendritic-cell vaccines are considered among the most successful approaches so far, as these cells can elicit strong immune responses (see text box).
Other vaccination approaches use whole tumor cells, DNA or RNA, peptides or proteins, as well as viruses (Kamta, et al., 2017). “There is a high interest in finding neoantigens, which arise from mutations within the cancer genome, and which can elicit a T cell response,” remarks Wölfel. “In recent years, new high throughput DNA-sequencing methods have allowed the identification of antigens in individual patients in an acceptable period of time. Nevertheless, the mean time of approximately three months might be too long for some patients in an advanced stage of the disease without other therapeutic options.” Despite researchers’ high expectations, early clinical studies of vaccines did not show the anticipated results, as cancer-mediated T cell suppression prevented T cell activation. Therefore, recent studies have actively focused on combining vaccination with other approaches such as T cell checkpoint inhibition (Patel et al., 2016). Improving vaccine design, and using vaccines to increase the effects of standard therapies could also maximize therapeutic benefits for cancer patients.
Dendritic cell based vaccines
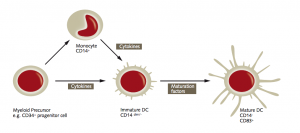
Dendritic cells (DCs) are the most powerful antigen-presenting cells in the human body. They take up foreign molecules, process them and present them to T cells starting an immune response. During the past decade, DCs have been the subject of numerous studies aimed at gaining a better insight into their immunobiology, their interaction with both the innate and adaptive immune system, and their role in the tumor microenvironment. In the context of cancer immunity, DCs can activate tumor-specific T cells in lymphatic tissues. This is why DCs can be used as adjuvants for vaccination, and why they hold great promise for the treatment of cancer (Shang, et al., 2017). Sipuleucel-T is so far the only FDA-approved DC-based vaccine. It is used for treating advanced prostate cancer, as it has shown a survival benefit for patients.“Dendritic cells are essential for inducing an immune reaction,” explains Prof. Dr. med. Thomas Wölfel from Internal Medicine III of the University Medical Center Mainz. “The question is how they should be used therapeutically. Should they be extracted from the body, loaded with the relevant antigen and then reinfused, or should they be activated in the body through so-called danger signals?”
Formulated in 1994 by Polly Matzinger, the danger model postulates that the immune system differentiates between “dangerous” and “safe” by recognizing alarm signals coming from injured tissues. These danger signals can activate DCs and initiate an immune response. If tumors do not send those signals, they remain invisible to the immune system.
For this reason, the efficacy of DC vaccines is strongly influenced by the method of administration. Low numbers of DCs at the tumor site, impaired DC maturation, or the presence of inhibitory signals can endanger the therapeutic efficacy of the vaccine. Ex vivo DCs are mainly generated from bone marrow progenitor cells in the presence of GM-CSF and IL-4 or IL-13 (Pizzurro et al., 2015). In vivo vaccination strategies, where DCs are directly activated at multiple sites, offer a cheaper and simpler approach. To optimize DC-based vaccination strategies, researchers are now focusing on determining the optimal tumor-associated antigens to be used, the best way of administration, as well as possible combination therapies.
Cell therapy: providing the immune system with the missing effectors
The aim of immune checkpoint inhibitors and cancer vaccines is to trigger pre-existing immune responses. Yet what happens if there are no or not enough effector cells to fight the tumor? To overcome this deficit, researchers can extract T cells from a patient’s blood or tumor samples, expand them in vitro, and reintroduce them into the patient as a cancer therapy. Steven Rosenberg had been working for years on the adoptive transfer of tumor infiltrating lymphocytes (TILs), when in 2010, he published encouraging results obtained with the so-called chimeric antigen receptor therapy (CAR). Here, a patient’s T cells are collected and genetically engineered so that they target cancer cells (June et al., 2018). CAR therapy holds significant promise for the future.
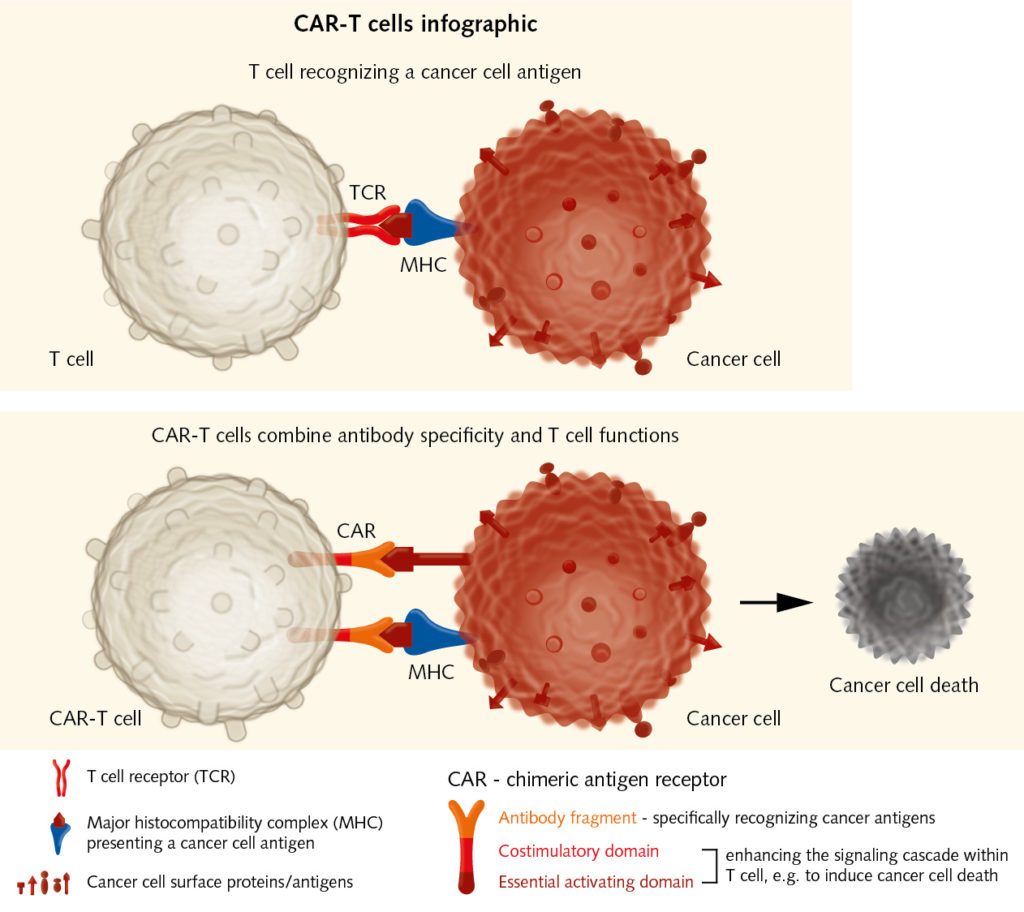
Although first used in hematological malignancies, recent studies have shown that adoptive cell transfer can also be used to treat other malignancies. The biggest challenge remains toxicity, which sometimes results in life-threatening immune reactions, as the infusion of billions of lymphocytes into the patient can trigger the release of cytokines. These mediators can cause fever, fatigue, cardiac, liver, and renal toxicity, and eventually organ failure. Genetic engineering could allow the use of TILs that have been sensitized to tumor-specific antigens, which would make the adoptive transfer safer and more effective. “The aim is transferring effectors with known specificity, but this is complex and still very expensive,” explains Wölfel. To improve efficacy, CAR therapy could also be combined with checkpoint inhibition, as the transferred T cells have to overcome the barriers set by the cancer cells as well.
Future challenges: finding the right approach for each patient
“Experience collected in the past three decades of immunotherapeutic research suggests that only by combining different strategies will we be able to offer cancer patients an effective and sustainable therapy,” says Wölfel. “We have to look for therapeutic algorithms, as well as predictive biomarkers, which are lacking at the moment.” Biomarkers could give researchers evidence on the immunological repertoire of an individual patient, on the immunogenicity of the tumor, and on the barriers that the tumor has put in place to defend itself. Understanding immune-escape mechanisms is crucial to designing personalized cancer immunotherapies, in which different strategies are chosen based on the specific patient’s biology (Kim and Chen, 2016). “There will be no magic bullet, nor cure-all approach. Immunotherapies have to be highly individualized and optimized for each patient,” concludes Wölfel.