The Human Leukocyte Antigen (HLA) system is a set of genes that play important roles in health and disease, serving as a flag for ‘self’ and ‘non-self’ cells. As such, HLA molecules have implications in several biomedical research areas, from immunology to transplantation and precision medicine. In this article, we explore what HLA typing is, why it matters, and how HLA research is shaping our understanding of autoimmune diseases, infectious diseases, stem cell transplantation, and cancer.
What is the HLA system?
The Human Leukocyte Antigen (HLA) system, also known as the major histocompatibility complex (MHC), is one of the most complex and variable regions of the human genome.1 The system encodes cell surface proteins that are essential for immune system regulation and antigen presentation. The HLA molecules are divided into class I (HLA-A, HLA-B, HLA-C) and class II (HLA-DR, HLA-DQ, HLA-DP).2
These molecules help the immune system distinguish between the body’s own cells and tissues (‘self’) and foreign substances such as pathogens and transplanted cells (‘non-self’), playing a pivotal role in immune response, disease susceptibility, and transplantation outcomes.1,2
What is HLA typing, and why is it important?
HLA typing is the process of identifying the specific HLA genes and alleles present in an individual. This information is critical for3–5:
- Matching organ and stem cell donors with recipients
- Understanding genetic predisposition to diseases
- Developing and testing immunotherapies
- Advancing research on HLA and its role in disease mechanisms
The HLA gene family is extremely diverse, with over 28,000 class I and more than 12,000 class II alleles identified in the human genome.6 This diversity makes HLA in research both fascinating and challenging.
The importance of HLA for the development of cell therapies
Interested in understanding the critical role of the HLA complex in human immunity?
What is the role of HLA in disease research?
HLA research has transformed our understanding of how immune cells interact with other cells in our body and how the immune system prevents diseases.
Autoimmune diseases
Research has revealed strong associations between specific HLA alleles and autoimmune diseases. Several HLA alleles have been found to predispose individuals to various autoimmune conditions:
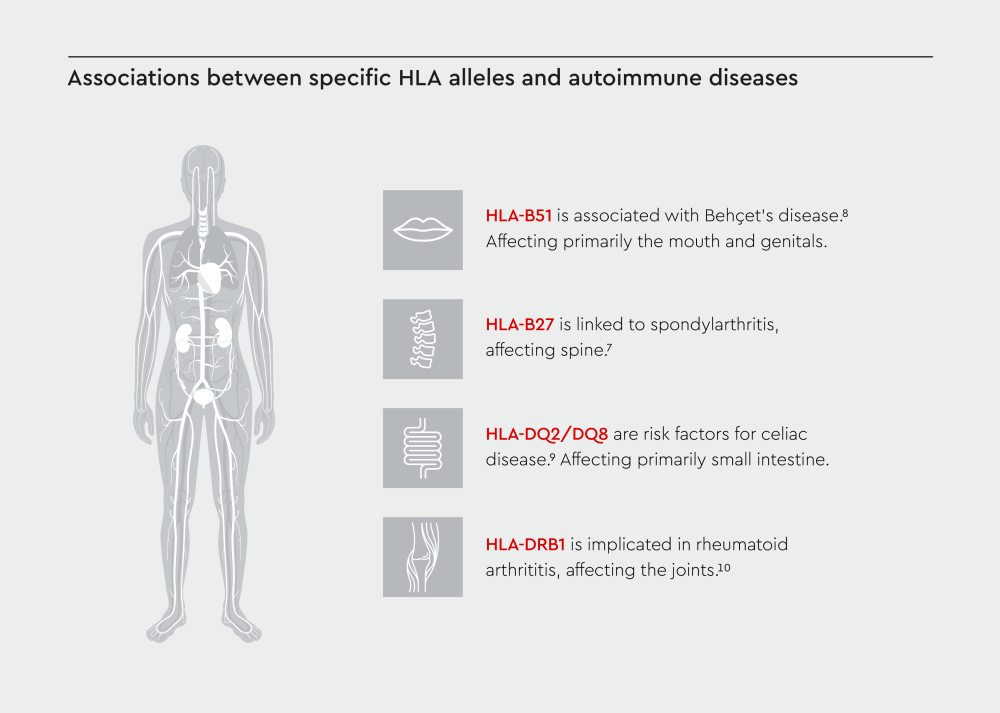

Figure 1: Visual representation of HLA types and their associated autoimmune conditions affecting different tissues and organs in the body.
These associations help researchers understand the mechanisms of immune system dysregulation and guide the development of diagnostic biomarkers and targeted therapies.11 HLA typing is now routinely used in the diagnostic workup for certain autoimmune conditions.
Infectious diseases
Research has shown that HLA molecules play a central role in how the body responds to infections. Certain HLA alleles can increase or decrease susceptibility to viral infections such as HIV, hepatitis B and C, and SARS-CoV-2.12,13
- HLA class I molecules present viral peptides to CD8+ T cells, driving cytotoxic responses.12
- HLA class II molecules activate CD4+ T cells, supporting antibody production and memory responses.12
Understanding these interactions enables the prediction of disease outcomes and the design of effective vaccines and therapies.
Stem cell transplantation
HLA research is vital for successful hematopoietic stem cell transplantation (HSCT). HLA matching between donor and recipient reduces the risk of graft rejection, graft-versus-host disease (GVHD), and other complications.14
Advances in HLA typing have improved donor selection, making transplantations safer and more effective. The number and type of mismatches affect transplant outcomes, and research on permissible mismatches and immune modulation is expanding donor options.14 Therefore, HLA-typed cells are essential for preclinical testing and cross-reactivity studies before transplantation.
Cancer and personalized immunotherapies
HLA research is driving breakthroughs in cancer immunotherapy. Tumor-reactive T cells recognize cancer cells through HLA molecules, and the effectiveness of therapies like adoptive cell transfer depends on the compatibility.15,16 HLA typing helps identify patients likely to respond to immunotherapies, and HLA-typed cells are used to test new cancer treatments and minimize off-target effects.17,18 In addition, certain alleles serve as biomarkers for predicting cancer treatment outcomes.17,19,20
What are the latest advances in HLA research?
The landscape of translational research is advancing rapidly, with several new developments in technology and methodology for developing personalized treatment and HLA-guided therapy. Human primary cells with known HLA types provide a physiologically relevant platform for drug testing and immunological studies. This offers an enhanced translational potential compared to animal models .21 These pre-typed cell models have become invaluable tools in various research applications.
Personalized treatment and HLA-guided therapy
Research has revealed strong associations between specific HLA alleles and autoimmune diseases. Several HLA alleles have been found to predispose individuals to various autoimmune conditions:
- Precision medicine: HLA typing enables the development of therapies tailored to an individual’s genetic makeup, improving efficacy and reducing side effects of treatments.5,22
- Immunogenetics: Identifying genetic predisposition to diseases guides the design of targeted interventions.4
- HLA-guided therapy: Therapies such as adoptive T cell transfer, checkpoint inhibitors, and peptide vaccines are increasingly designed based on HLA genotype.23,24
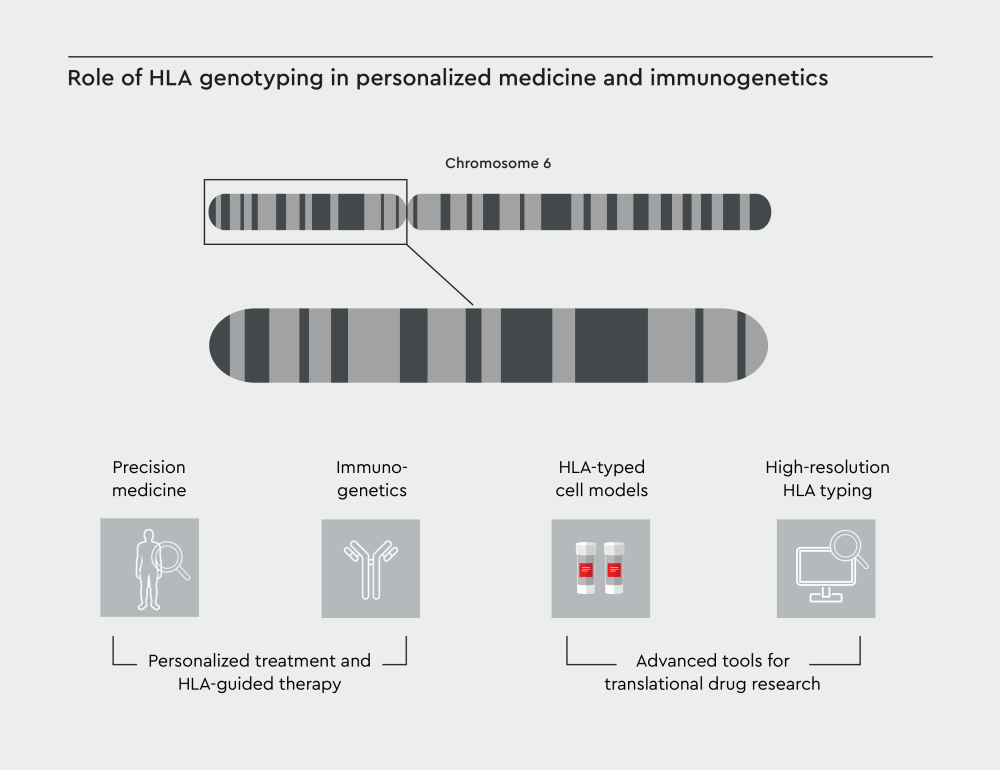

Figure 2: Advanced tools, such as high-resolution HLA typing and HLA-typed cell models, can enhance translational drug research. The integration of HLA-informed approaches enhances therapeutic efficacy while minimizing side effects.
Challenges in HLA research
The diversity and complexity of the HLA system create several challenges. The system is extremely polymorphic, with thousands of HLA alleles.6 In addition, HLA molecules vary in peptide-binding properties, affecting immune responses.4
This genetic complexity and structural diversity complicate typing and matching. Accurate and high-throughput HLA typing requires advanced technology and expertise, which can be costly.25 Moreover, traditional cell models often lack the diversity and specificity needed to study HLA-driven responses.26
- Human relevance: Pre-typed primary cells mimic the human immune environment, enabling more translational research.
- Diversity: Large inventories of HLA-typed cells allow for the study of rare alleles and population-specific responses.
- Efficiency: Pre-typed cells save time and resources, accelerating research and development.
Future directions
There are several emerging trends and methodologies in HLA research, with HLA-typed cells becoming increasingly vital tools for researchers.
Current and emerging applications of HLA-typed cells
- Immunotherapies: They enable precise testing of T cell-based therapies and immune checkpoint inhibitors, allowing researchers to predict responses across diverse patient populations.23,27
- Transplantation: These cells facilitate in vitro compatibility testing, helping refine matching protocols and develop strategies to overcome HLA mismatch.28
- Vaccine development: Using HLA-typed cells allows for the identification and validation of epitopes that can trigger immune responses across multiple HLA types, leading to more universally effective vaccines.27
- Drug screening and toxicity testing: They provide a more accurate prediction of immune-related adverse events and drug hypersensitivity reactions than traditional models.29
Standardized protocols and translational research
- Integration of HLA-typed cells: Standardized protocols are being developed to ensure reproducibility and reliability in translational research.30,31
- Collaborative databases: Sharing data through initiatives such as the IPD-IMGT/HLA Database6 accelerates discovery and application across the biomedical community.
How can we support your HLA research?
We are committed to empowering you with the tools and expertise you need to advance your research. Here’s how we can help you:
- High-quality HLA-typed cells: Choose from a broad range of HLA-typed human primary cells and PBMCs from over 100 donors, with complete 4-digit resolution typing for HLA-A, HLA-B, HLA-C, HLA-DPA1/DPB1, HLA-DQA1/DQB1, and HLA-DRB1/DRB345.
- Customized cell culture media: Our specialized media robust cell growth and function, ensuring reliable results.
- Expert scientific support: Our cell culture specialists are ready to assist you with protocol optimization, troubleshooting, and custom solutions for your research.
References
Expand
- Trowsdale J, Knight JC. Major histocompatibility complex genomics and human disease. Annu Rev Genom Hum Genet. 2013;14(1):301–323. doi:10.1146/annurev-genom-091212-153455
- Liu B, Shao Y, Fu R. Current research status of HLA in immune‐related diseases. Immunity Inflam & Disease. 2021;9(2):340–350. doi:10.1002/iid3.416
- Choo SY. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Med J. 2007;48(1):11–23. doi:10.3349/ymj.2007.48.1.11
- Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol. 2018;18(5):325–339. doi:10.1038/nri.2017.143
- Jin P, Wang E. Polymorphism in clinical immunology – From HLA typing to immunogenetic profiling. J Transl Med. 2003;1(1):8. doi:10.1186/1479-5876-1-8
- European Molecular Biology Laboratory. IPD-IMGT/HLA Database. Accessed April 29, 2025. https://www.ebi.ac.uk/ipd/imgt/hla/about/statistics/
- Ramos M, De Castro JAL. HLA-B27 and the pathogenesis of spondyloarthritis. Tissue Antigens. 2002;60(3):191–205. doi:10.1034/j.1399-0039.2002.600301.x
- Mizuki Y, Horita N, Horie Y, et al. The influence of HLA-B51 on clinical manifestations among Japanese patients with Behçet’s disease: A nationwide survey. Modern Rheumatology. 2020;30(4):708–714. doi:10.1080/14397595.2019.1649103
- Aboulaghras S, Piancatelli D, Taghzouti K, et al. Meta-analysis and systematic review of HLA DQ2/DQ8 in adults with celiac disease. Int J Mol Sci. 2023;24(2):1188. doi:10.3390/ijms24021188
- Gonzalez-Gay MA, Garcia-Porrua C, Hajeer AH. Influence of human leukocyte antigen-DRB1 on the susceptibility and severity of rheumatoid arthritis. Semin Arthritis Rheum. 2002;31(6):355–360. doi:10.1053/sarh.2002.32552
- Bodis G, Toth V, Schwarting A. Role of human leukocyte antigens (HLA) in autoimmune diseases. Rheumatol Ther. 2018;5(1):5–20. doi:10.1007/s40744-018-0100-z
- Blackwell JM, Jamieson SE, Burgner D. HLA and infectious diseases. Clin Microbiol Rev. 2009;22(2):370–385. doi:10.1128/cmr.00048-08
- Medhasi S, Chantratita N. Human leukocyte antigen (HLA) system: genetics and association with bacterial and viral infections. J Immunol Res. 2022;2022(1):9710376. doi:10.1155/2022/9710376
- Park M, Seo JJ. Role of HLA in hematopoietic stem cell transplantation. Bone Marrow Res. 2012;2012:680841. doi:10.1155/2012/680841
- Lowery FJ, Krishna S, Yossef R, et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022;375(6583):877–884. doi:10.1126/science.abl5447
- Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi:10.1038/nrc1167
- Chowell D, Morris LGT, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359(6375):582–587. doi:10.1126/science.aao4572
- van Loenen MM, de Boer R, Amir AL, et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci USA. 2010;107(24):10972–10977. doi:10.1073/pnas.1005802107
- Naranbhai V, Viard M, Dean M, et al. HLA-A*03 and response to immune checkpoint blockade in cancer: an epidemiological biomarker study. Lancet Oncol. 2022;23(1):172–184. doi:10.1016/S1470-2045(21)00582-9
- van Belzen IAEM, Kesmir C. Immune biomarkers for predicting response to adoptive cell transfer as cancer treatment. Immunogenetics. 2019;71(2):71–86. doi:10.1007/s00251-018-1083-1
- Faulkner L, Gibson A, Sullivan A, et al. Detection of primary T cell responses to drugs and chemicals in HLA-typed volunteers: implications for the prediction of drug immunogenicity. Toxicol Sci. 2016;154(2):416–429. doi:10.1093/toxsci/kfw177
- Geo JA, Ameen R, Al Shemmari S, Thomas J. Advancements in HLA typing techniques and their impact on transplantation medicine. Med Princ Pract. 2024;33(3):215–231. doi:10.1159/000538176
- Couture A, Garnier A, Docagne F, et al. HLA-class II artificial antigen presenting cells in CD4+ T cell-based immunotherapy. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.01081
- Faghfuri E. Recent advances in personalized cancer immunotherapy with immune checkpoint inhibitors, T cells and vaccines. Pers Med. 2024;21(1):45–57. doi:10.2217/pme-2023-0054
- Bravo-Egana V, Sanders H, Chitnis N. New challenges, new opportunities: next generation sequencing and its place in the advancement of HLA typing. Hum Immunol. 2021;82(7):478–487. doi:10.1016/j.humimm.2021.01.010
- Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13(11):1285–1306. doi:10.2217/pgs.12.108
- Lee MN, Meyerson M. Antigen identification for HLA class I– and HLA class II–restricted T cell receptors using cytokine-capturing antigen-presenting cells. Sci Immunol. 2021;6(55):eabf4001. doi:10.1126/sciimmunol.abf4001
- Mayor NP, Hayhurst JD, Turner TR, et al. Recipients receiving better HLA-matched hematopoietic cell transplantation grafts, uncovered by a novel HLA typing method, have superior survival: A retrospective study. Biol Blood Marrow Transplant. 2019;25(3):443–450. doi:10.1016/j.bbmt.2018.12.768
- Di Zeo-Sánchez DE, Segovia-Zafra A, Matilla-Cabello G, et al. Modeling drug-induced liver injury: current status and future prospects. Expert Opin Drug Metab Toxicol. 2022;18(9):555–573. doi:10.1080/17425255.2022.2122810
- Alvarez-Palomo B, Vives J, Casaroli-Marano RP, et al. Adapting cord blood collection and banking standard operating procedures for HLA-homozygous induced pluripotent stem cells production and banking for clinical application. J Clin Med. 2019;8(4):476. doi:10.3390/jcm8040476
- Edgerly CH, Weimer ET. The past, present, and future of HLA typing in transplantation. In: Boegel S, ed. HLA Typing: Methods and Protocols. Springer; 2018:1–10. doi:10.1007/978-1-4939-8546-3_1


Interested in learning more? Discover our MSC eBook
Explore key insights into MSC cell culture, emphasizing the importance of media selection for cell therapy applications.
Related resources